
There are many different methods used in today’s laboratories to assist in the concentration and/or drying of samples. Many of these methods use a combination of techniques, such as temperature increase and vapor pressure reduction, to expedite the solvent evaporation process. For example, Organomation’s N-EVAP nitrogen evaporators combine nitrogen blow down with a heated bath to accelerate concentration. In these and other atmospheric gas flow assisted evaporation systems, the evaporation rate of a particular solvent is dependent on temperature and gas flow.
It is an established principle of evaporative systems that the evaporation rate increases as the temperature approaches the boiling point of the solvent. Unfortunately, in many cases the heat needed for the removal of reaction solvents and diluents may degrade or even destroy a sample or analyte. As a result, it is nearly always advantageous to minimize the evaporation temperature.
At the same time, the evaporation rate of a solvent at a fixed temperature generally increases proportionally with the gas flow rate directed onto the sample’s surface. Unfortunately, it is also recognized that a higher gas flow rate generates more turbulence in the sample, which may cause spattering leading to sample loss and/or cross contamination.
To prevent sample degradation, the safest course of action utilizes the lowest temperature and gas flow possible in order to protect the sample integrity. Unfortunately, this approach increases evaporation time; and in the modern laboratory time is costly and is a severe obstacle to scientific progress. Thus, the ideal solution is to balance the temperature and gas flow rate to obtain the highest evaporation rate, while minimizing thermal degradation and excessive turbulence. But, how does one identify and achieve the optimal balance?
First, care should be taken in selecting the reaction and extraction solvents or diluents to ensure that the boiling point of the solvent will not result in thermal degradation or sample loss due to partial volatility during the evaporation process. As a guideline, most evaporation procedures are conducted at a temperature approximately 10 degrees Celsius below the boiling point of the subject solvent. This is generally safe with respect to thermal degradation of the sample or analyte because the same solvent is often used throughout the synthesis, processing and/or isolation of the sample or analyte.
In a gas assisted evaporation process, the gas - nitrogen, compressed air, etc. - is channeled through a manifold at a selected flow rate which is controlled by a pressure regulator, gas flow meter, and/or a needle valve. The gas stream flows through a needle or nozzle which creates a gas jet that is directed onto the solvent/sample surface. At a set flow rate, the gas flow through the needle or nozzle is constant. Therefore, the gas jet velocity increases with decreasing needle size, i.e. a smaller needle gauge creates a higher gas jet velocity. When samples with a volume of 10 – 40 ml and an outside diameter (OD) of ≤25 mm are being processed, a smaller gauge needle (19 gauge) is more effective than a larger gauge needle (10 gauge). This relationship is clearly seen in the evaporation of acetone in a 16mm test tube (Figure 1).
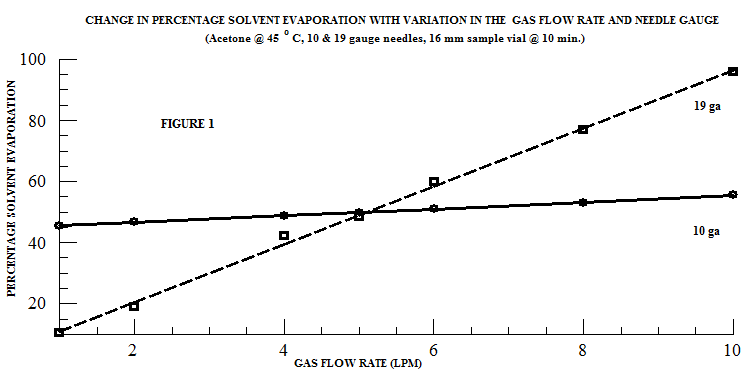
However, this principle does not apply to all samples. An additional factor, often overlooked when working with gas flow assisted evaporation systems, is sample size. Larger samples require larger vials which may affect the evaporation rate through the introduction of variables such as surface to volume ratio, turbulent flow restrictions, and condensation. These effects are caused by the increased surface area of the sample vial in larger samples.
In larger samples, there is typically a larger volume of solvent and, consequently, the amount of solvent vapor that must be removed is larger. Subsequently, the physical removal of this larger volume of solvent vapor from the sample requires a larger gas volume. Unlike in the 16 mm OD sample vial (Figure 1), in the sample vial with an outside diameter of 48 mm - containing 200 ml of solvent - a significantly different relationship between needle gauge and evaporation rate is observed: at a fixed temperature and gas flow rate, the evaporation rate increases with increasing needles aperture (Figure 2). Thus, in large samples, a larger needle aperture increases the evaporation rate by directing a greater gas volume onto the sample’s surface. Further, at constant temperature and a fixed needle gauge, the evaporation rate increases with increases in the gas flow rate (Figure 3).
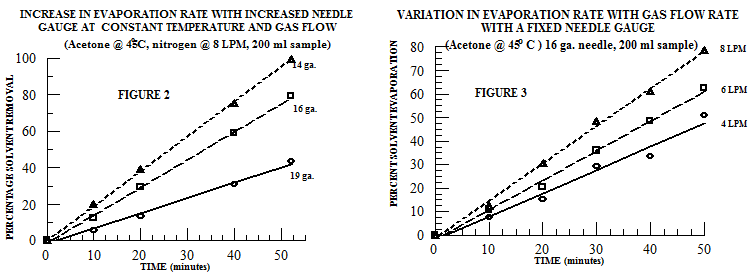
The general trends discussed here are helpful guidelines to use when determining solvent evaporation parameters. However, the best combination of needle size, temperature, and gas flow volume/velocity will vary with sample size, sample holder configuration, and the specific solvent being removed. Ultimately, experience and experimental evaluation are the key to selecting the most efficient and effective system for solvent removal.